Medical research company plays important role in saving lives
By Cheryl Long
The gains of scientific research came full circle for interventional cardiologist Dr. Guy Leclerc when the new cardiac valve that his company, AccelLAB, tested in preclinical studies was finally approved for use in a human patient. And as it turned out, one of Dr. Leclerc’s colleagues, Dr. Jeannot Potvin, the cardiologist who performed the preclinical study of the cardiac valve in a swine model at AccelLAB, was also the one who successfully implanted the new valve in the first North American patient at the CHUM (Centre Hospitalier de l’Université de Montreal).
That’s the ultimate success for Leclerc and Accel LAB — helping to prove through research that a new implant or device has the potential to improve the quality of life for human patients worldwide.
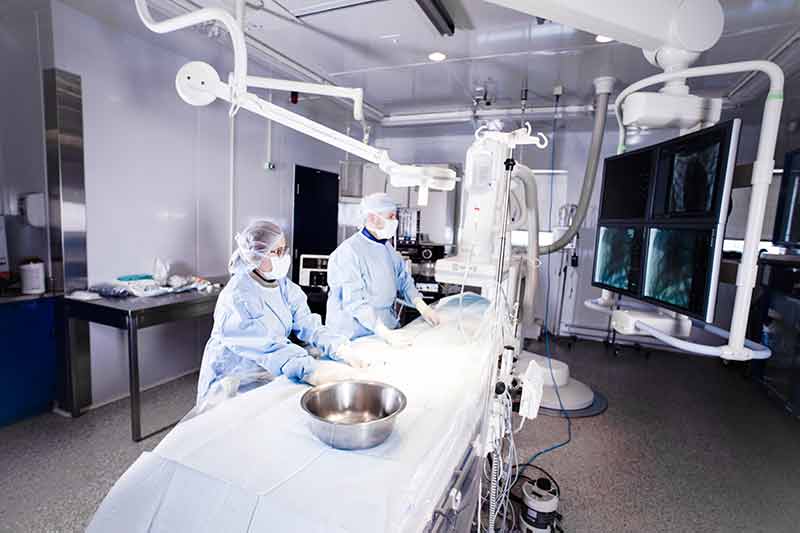
That research takes place at AccelLAB, located in Boisbriand, Quebec, a suburb 30 minutes north of downtown Montreal. Leclerc is CEO and founder of the company that is contracted to conduct the early stages of research in the field of medical devices and biotechnology. Whether it’s cardiology, orthopedics or regenerative medicine, AccelLAB takes new inventions created by manufacturers around the world and tests their safety and efficacy in animals, collecting data that can be used to prove the device’s suitability for use in human patients. Once proven, the device may then be approved for clinical trials in humans and eventually enter the health care market.
Previously the owner of a biotechnology company, Leclerc founded AccelLAB in 2004 and soon identified a group of nine people equipped with the skills and knowledge to join him in his new venture. The solidity of the company and its mission are evident in AccelLAB’s retention rate — seven of those original team members are still with the company. Today, AccelLAB has more than 90 employees who demonstrate a keen level of commitment.
“Even with our large numbers of employees, I’m proud to say that when we do hire people they always stay on board because I do believe we offer them a challenging job with great working conditions,” Leclerc said. “We’re able to do it because we’re still relatively small, although we’re growing. You can’t build something strong if you don’t have a strong team that believes in it.”
AccelLAB is considered a preclinical contract research organization or CRO, and its offices and research facilities include a hybrid operating suite, two state-of-the-art cardiac catheterization labs, specialized laboratories with the latest in imaging technology, and histopathology and clinical pathology labs. They also operate a vivarium designed for preclinical studies, which helps to keep the animals used in their research in a healthy environment. Animals are also housed in short- and long-term facilities that comply with the highest industry standards, including the Organization for Economic Co-operation and Development (OECD) Good Laboratory Practice (GLP) initiative by the Standards Council of Canada. AccelLAB is approved by several regulatory agencies, including Health Canada and the FDA in the United States, and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and the Canadian Council on Animal Care (CCAC).
Those accreditations are vitally important for an organization like AccelLAB at a time when the focus on animal health and welfare, particularly in the area of medical testing, is closely scrutinized worldwide.
“It’s an environment that’s very highly regulated. I think we’ve seen a lot of refinements even over the past few years,” Leclerc explained. “We’re being audited by agencies like CCAC and also the American AAALAC. Through these audits we’re constantly improving our methods and sharing that with other users with similar methodology. There’s a constant improvement in this field of research.”
In an effort to ensure the health and welfare of the animals in their program, AccelLAB operates a veterinary care, preventive medicine and biosecurity program run by attending veterinarians and animal care technicians. All animals are monitored daily, year-round. And while testing on animals is vital to advances in medical care for human patients, AccelLAB still strives to replace animals with non-animal models in their research, Leclerc said, hoping to reduce the number of animals used in their studies.
“There’s a limit to what can be done on a bench test without a live system,” he explained, “and that’s why we do need to test these new implants that do save lives, that do improve the quality of life of humans. We do need to test these devices in live systems such as animals.”
One advance that AccelLAB has been testing is a cardiac stent that is designed to be absorbable by the body. Current stents are made of metal and stay within a vessel or artery permanently unless removed. Medicine is starting to shift away from permanent stents, realizing that over time vessels can recover their ability to function normally, eliminating the need for the metal stents that essentially “imprison” the vessel, Leclerc explained. “The industry has taken a very significant turn towards developing these absorbable devices. Millions of dollars are being invested into clinical research in that field.”
There are also new approaches being developed that allow surgeons to implant a stent or cardiac valve using less invasive methods that avoid opening up the chest. “Even though I see these procedures on a regular basis, I’m still impressed by the technology,” Leclerc said.
Most of AccelLAB’s competition in the preclinical testing of medical device market can be found in the U.S. and Europe. In Canada, the company is “very focused and niched”, Leclerc said, and the barrier to entry into the specialized market is high due to the investment required in both capital and expertise. Currently, AccelLAB has a presence in North America, Europe and Asia, and is very much an international player in its sector. Last year, the Quebec Chamber of Commerce awarded AccelLAB with the Mercuriades Award in the Export and International Market Development category. The prestigious annual business awards honour the outstanding achievements of Quebec companies as a source of inspiration for the province’s future.
“It is nice recognition for the whole team,” Leclerc said. “For the first time a completely independent jury acknowledged that we’re on top of things.”
Long term, Leclerc would like to see AccelLAB continue to grow by delving into areas of medical research that are new for the company, including ophthalmology, dental, and ear, nose and throat medicine. They’ll continue to offer the best quality in the industry, which is one of the keys to the company’s success.
“There is a race in the field of medicine for saving lives and improving quality of life. You have to be very diligent. You have to do this at a reasonable price. I think that at the end of the day, our biggest key element that has enabled us to reach this success is our employees. We do recognize that without them, we wouldn’t be where we are now.” To learn more about AccelLAB, visit accellab.com.